Discover our range of products
Van Raam produces uniquely special needs bicycles and specializes in tricycles, transport bikes, scooterbikes, wheelchair bikes, tandem bikes, double rider bikes, and low step trough bikes (also known as comfortbikes). Each model is also available as an electric bike (Pedelec).
About vanRaam
vanRaam is a Dutch producer of unique custom bikes. Want to know more about our company or special needs bikes?
Menu
Search
Our bikes
Quality Labels
At vanRaam, we place the utmost importance on the quality of our bikes. It is for this reason that we possess a number of quality labels and are members of the Firevaned branch association. We are also very proud of the various awards and honours we have received in recent years.
Quality Labels and Affiliated Organisations
Quality is our highest priority. vanRaam possesses several important quality marks and can therefore guarantee quality. vanRaam is also affiliated with the branch association and we continue to innovate thanks to the innovation hub and Smart Industry.
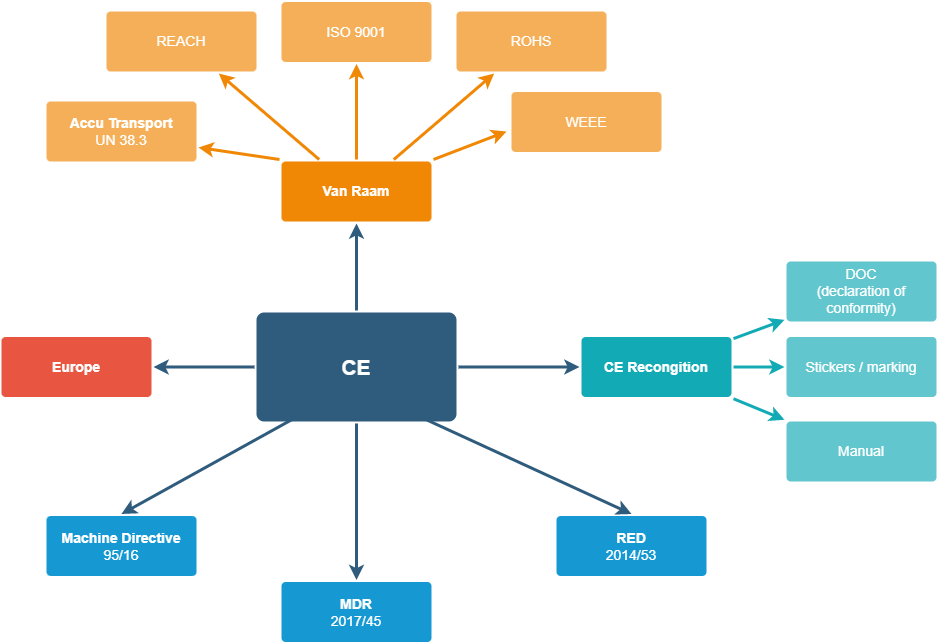
See below the schematic overview of all standards and legislation that vanRaam complies with. The basis is the ce-declaration:

CE-declaration
ISO 9001
Machinery Directive
Radio Equipment Directive (RED)
Member of Firevaned (National Services) branch organisation
Nationaal Hulpmiddelen (National Services)
Code of Conduct for Medical Devices
MDR Legislation
Innovar innovation hub
Member of Smart Industry
Step 3 on the Social Entrepreneurship Performance Ladder (PSO)
1. CE-declaration
vanRaam bicycles are delivered with a CE-declaration. The CE-declaration states which European standards and guidelines the products comply with.
2. ISO 9001
Quality comes first at vanRaam! We produce Dutch quality bicycles for adults and children. To demonstrate that we meet various quality requirements, we are ISO 9001:2015 certified.
ISO (International Organization for Standardization) is an international organization that sets standards. ISO 9001 is a globally recognized standard with requirements in the field of quality management. It contains requirements for companies to manage and improve the quality of their products and services. The standard also provides guidelines for the continuous improvement of customer satisfaction.
3. Machinery Directive
The Machinery Directive consists of requirements for machinery placed on the market within the European Union. These health and safety requirements are thus applicable to machinery in the design phase and to machinery produced for the first time. The Machinery Directive also covers powered, rolling machines, such as bicycles for example. We comply with the Machinery Directive with our machines and bicycles.
4. RED
The Radio Equipment Directive (RED) applies to all equipment with a built-in transmitter or receiver that uses the radio spectrum. Radio equipment includes radios, televisions, cell phones, wireless wifi equipment and all kinds of 'smart' equipment, such as our electric bikes. It must ensure that the equipment meets essential health and safety requirements and does not cause interference to other equipment. Due to the telematics module in our electrical system, we have to deal with this directive, which we comply with.
National Services
5. vanRaam is a member of the Firevaned branch organisation
Firevaned is a branch organisation for rehabilitation and mobility aids.
6. Nationaal Hulpmiddelen (National Services) quality label
Firevaned, the branch organisation for rehabilitation and mobility aids, has taken the initiative to set up an independent label. In 2014, the Nationaal Keurmerk Hulpmiddelen (NKH, National Services Quality Label) was launched.
With this label, companies can demonstrate that they do the utmost to ensure service, quality and accessibility. As a producer of special needs bikes, vanRaam is a member of Firevaned and collaborates on the joint mission to build a healthy industry.

7. Code of Conduct for Medical Devices
vanRaam has, as a supplier of medical devices, signed and is obliged to comply with the Code of Conduct Medical Devices. The Code of Conduct was drawn up between suppliers of medical devices and the parties involved in the decision-making process regarding the purchase and/or application thereof. The rules of conduct drawn up for this purpose ensure transparency and responsible dealings between both parties. By signing the code of conduct, suppliers, healthcare professionals and healthcare institutions commit themselves to compliance with the rules of conduct laid down in it. These are based on reciprocity; what one party may not offer or give, the other party may not ask or accept.
8. MDR legislation
MDR is the new Medical Devices Regulation, which has replaced the former MDD (Medical Devices Directive) in Europe.
One of the most important parts in this law is that medical devices are better traceable in the whole chain. For this purpose, the unique number of the bicycle has been expanded to a so-called UDI, Unique Device Identification. The necessary GS1 barcodes are available for this, they must actually be visible on the product of vanRaam as of May 26, 2025. This date applies to products in class 1, such as vanRaam's bicycles.
The Basic-UDI-DI is from May 26, 2021 onwards included on the declaration of conformity. Each product group, as for example Easy Rider 3 or Fun2Go, has its own Basic-UDI-DI. This Basic-UDI-DI in combination with the frame number, the Production Identifier (PI), on the bicycle forms the unique number as required in the MDR. The frame number has always been applied to the bicycle by vanRaam using a barcode and is a unique number for each bicycle produced. The barcode on the declaration of conformity and the frame number on the bicycle together form the UDI.
In addition to these codes, the MDR also requires Post Market Surveillance and Clinical Investigation to be carried out. This means that research must be carried out to determine whether the products that vanRaam puts on the market actually correspond to the medical nature that the product is supposed to support.
9. Innovar Innovation Hub
vanRaam is initiator and participant of the Innovation Hub Innovar which started in 2012. Innovar is a collaboration between three companies from Varsseveld, vanRaam, Contour and Waterkracht. Interns who go through Innovar to intern within one of these companies get extra guidance from a Hub Manager.
10. Member of Smart Industry East and BOOST Head Group
Many companies are currently involved in Smart Industry. However, only 5 companies - including vanRaam - form the 'BOOST Head Group'. BOOST is the action agenda for Smart Industry in the East Netherlands and makes the East Netherlands ready for Smart Industry.
They are a source of knowledge and inspiration for other entrepreneurs in the field of Smart Industry. They have created an Action Agenda with which they help integrate smart industry into the East Netherlands business culture.
Step 3 on the Social Enterprise Performance Ladder (PSO)
vanRaam is positioned at Step 3 on the Social Enterprise Performance Ladder (PSO). This is the highest level of the PSO and demonstrates that, either directly or indirectly, more people with a distance to the labour market are employed at vanRaam than at comparable organisations. Suppliers and contractors are also actively encouraged to engage in social entrepreneurship. The PSO is an independent certification and a scientifically validated measurement tool developed by TNO.

Do you have any questions?
We are happy to assist you! Please check our contact page or contact us directly using the options below.